Endometriosis is a benign disease affecting 10% of women of reproductive age. It is characterized by ectopic extra-uterine deposits of endometrial tissue, with the pelvic cavity being the most affected location. Abdominal wall endometriosis (AWE) is a relatively rare type of extrapelvic endometriosis, often occurring in patients with a history of a caesarean section or abdominopelvic surgery. AWE can be located within all layers of the anterior abdominal wall, including the subcutaneous tissue, the pre-fascial area, or the muscular layers (i.e. rectus abdominis).
Clinically, AWE may be silent, or may become symptomatic under cyclical hormonal stimulation, typically 3-5 years after a caesarean section or other abdominopelvic surgery. When clinically active, AWE presents as a painful palpable mass, which is often debilitating and has a significant negative impact on patients’ quality of life.
Historical treatments for AWE include analgesics, medical therapies aiming at blocking the hormonal stimulation to the target lesion, and surgical resection. These treatments are not tolerated by all patients. Moreover, many of them may refuse these options, and many others may feel these proposed treatments are too “heavy” for the benign profile of the disease. Surgical removal of the AWE in young patients with a previous history of abdominopelvic surgery may necessitate prosthetic implantation in up to 15% of patients.
Accordingly, there has been a substantial need for a minimally invasive alternative to the aforementioned traditional treatments. The proposed treatment needs to be effective in achieving long-lasting pain relief and, most of all, needs to be highly safe, given the young and healthy profile of the target population. In this perspective, cryoablation has succeeded in becoming a “reference”, and some technical aspects have without doubt contributed to this achievement. Among the most relevant aspects, we may list:
- The high analgesic profile of the iceball, which allows treatment under local anesthesia +/- mild sedation in most patients; and makes the post-operative phase very “smooth” for patients;
- The possibility of providing percutaneous treatment on an outpatient basis in most cases;
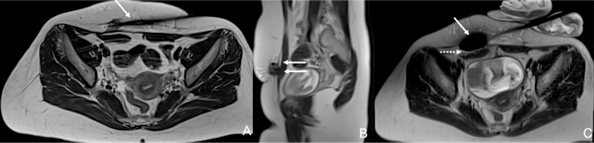
- The use of imaging (US and cross-sectional) to guide the intervention, which allows precise destruction of the AWE without provoking significant iatrogenic lesions to the surrounding non-target structures, mainly due to the intense use of ancillary protective measures (Fig .1).
Along with the aforementioned advantages, the clinical results available so far for cryoablation are very impressive, and this has probably contributed to the fast adoption of the technique. In fact, in a recently published metanalysis study, the difference between the visual analogue scores (VAS) before and after treatment was on average 5.97 points, the satisfaction rate among patients was 93.1%, and the prevalence of adverse events was 5.48%.
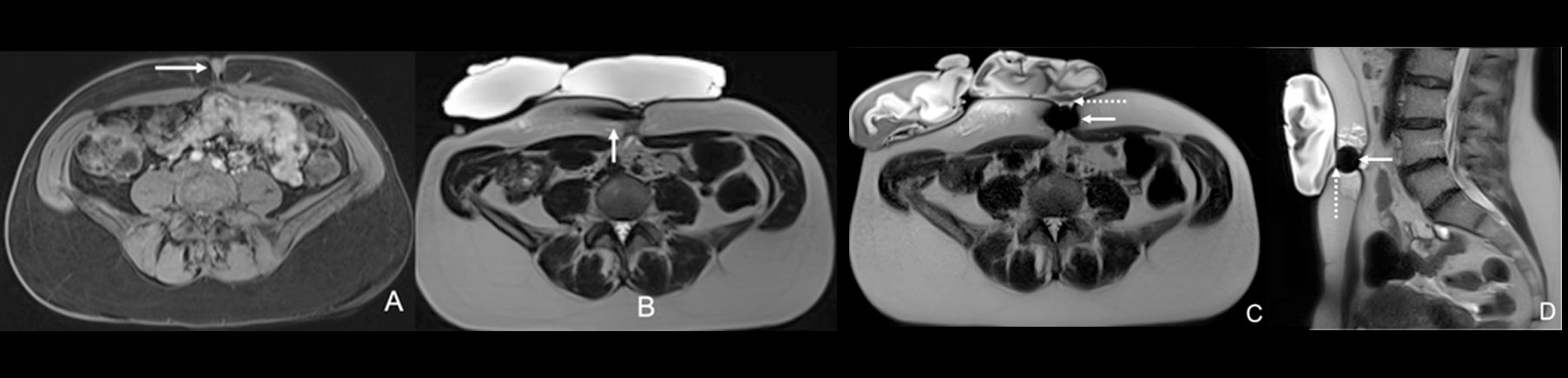
Based on these encouraging premises, interventionalists have recently started to push the boundaries by treating painful endometriosis deposits which are outside the abdominal wall and/or are located in challenging and unusual anatomic sites (i.e. umbilicus, inguinal canal, diaphragm, etc.) (Fig. 2). A recently published retrospective case-series showed that cryoablation is safe and effective when proposed in these challenging/unusual locations with reported rates of primary/secondary complete pain relief and adverse events respectively at 86.7%, 93.3% and 12.5%.
In conclusion, cryoablation represents a safe and effective option for painful AWE. In the near future, this technique is likely to “expand” in anatomic areas other than the anterior abdominal wall.